Summary, sample questions and discussion of acids, bases and buffers
Equation water ionizationH2O <=> H+ + OH‾
From the above reaction according to the law of equilibrium, the equilibrium constant (K) is written as follows
K [H2O] = [H+] [OH‾]
Kw = [H+] [OH‾]
at a temperature of 25 ° C obtained price Kw = 1.0 x 10-14
This means that at a temperature of 25 ° C in one liter of pure water contained 10-7 10-7 H + and OH ~ ions.
Example Problem 1
What is the concentration of H + and OH-in 500 mL of 0.1 M HCl solution?
answer
HCl(aq) → H+(aq) + Cl–(aq)
comparison of coefficients = 1 : 1 : 1
The concentration of OH-in a 0.1 M HCl is
[H+] [OH–] = 10–14 M
0,1 M [OH–] = 10–14 M
Example question 2
What is the concentration of H + ions and SO42-ions in 500 mL of 0.2 M H2SO4 solution?
answer
H2SO4(aq) → 2H+(aq) + SO42–(aq)
comparison of coefficients = 1 : 2 : 1
Example question 3
What is the concentration of OH-and H + in a solution of 0.2 M NaOH?
answer
NaOH(aq) → Na+(aq) + OH–(aq)
Comparison coefficient = 1 : 1 : 1
[H+] [OH–] = 10–14 M
[H+] x 0,2 = 10–14 M
Ionization constants Acid Tongue
Strong acids and bases completely ionized so it does not have the equilibrium constant. However, weak acids and bases have the equilibrium constant for the water only biodegradable or only partially ionized. For example, a weak acid (HA) is dissolved in water it decomposes according to the equation below.
HA(aq) <==> H+(aq) + A–(aq)
[H+] = [A–] maka
[H+]2 = Ka.[HA]
In the same way
the acid ionization constant Ka =
Ca = concentration of the initial acid
Kb = base ionization constants
Cb = concentration bases.
From the formula above, the concentration of H + and OH-from a weak acid and weak base price determined Ka and Kb origin unknown.
Relationships Ka, Kb with degrees ionization of acids and bases
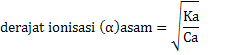
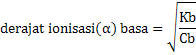
Ka = Ca x α2
Kb = Cb x α2
If Ka and Kb substituted into the formula
Be obtained following equation
Examples of working out how to calculate the concentration of H +
Determine the [H +] present in formic acid 0.01 M. If known Ka. HCOOH = 1.7 x 10-4.
answer
Ionization reaction HCOOH
HCOOH(aq) <==> H+(aq) + HCOO–(aq)





0 komentar
Post a Comment